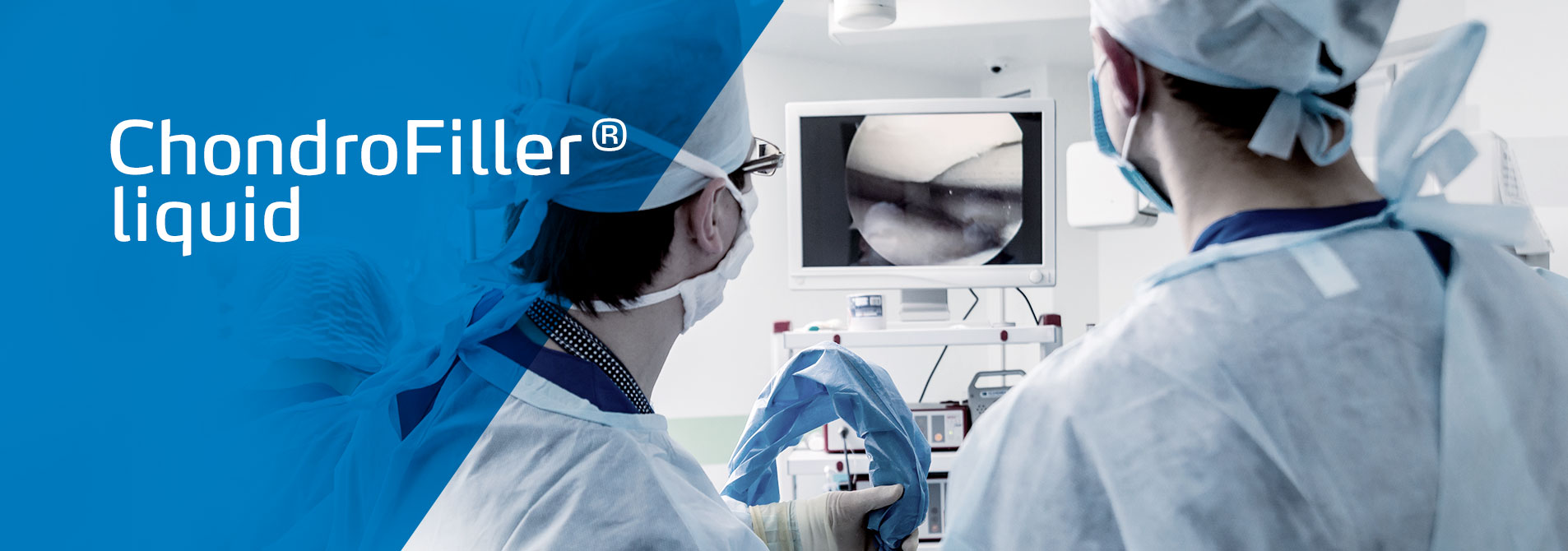
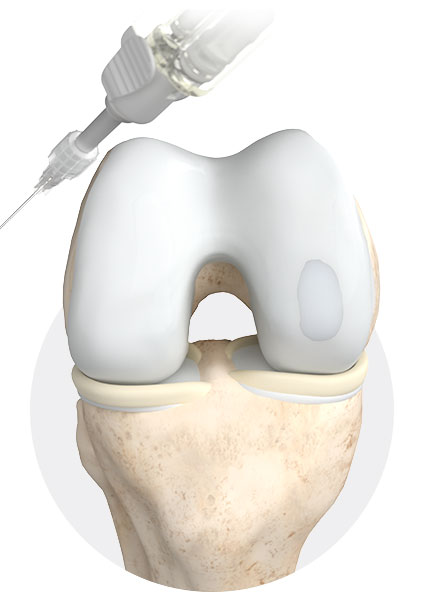
ChondroFiller® liquid is used to treat cartilage lesions gently, effectively and cost-effectively.
ChondroFiller® liquid is a biological, cell-free collagen matrix that has been specially developed to stimulate cartilage regeneration in joints. Just one arthroscopic procedure is required to implant the liquid in the target area. ChondroFiller® liquid is supplied in a ready-to-use two-chamber syringe. In addition to an ultrapure, native collagen type I solution, this also contains a neutralisation solution. When they are pressed out of the syringe, the two solutions are mixed with each other via a mixing adapter . After injection into the defect zone, the viscous collagen matrix hardens in approximately 3 to 5 minutes into a hydrogel, a dimensionally stable implant matrix. Even difficult to reach lesions, tiny tears and cavities can be filled with ChondroFiller® liquid. As a result, there is no need to use fibrin glue, and likewise there is no need for drilling into the bone (microfracturing).
Our medical devices are subject to strict quality requirements and are manufactured in Germany.
The advantages of treatment with ChondroFiller® liquid at a glance:
ChondroFiller® liquid varies in terms of shape, thickness and size. Immediately after injection into the defect zone a dimensionally stable implant matrix forms in approximately 3 – 5 minutes. Even difficult to reach lesions, tiny tears and cavities can be filled with ChondroFiller® liquid.
- Just one arthroscopic procedure is required.
- Surgery time is short, reducing the risks associated with surgery.
- The material is acellular, so no additional biopsy is required.
- The collagen matrix adapts to the individual size and shape of the cartilage defect.
- No fibrin glue is required – ChondroFiller liquid turns into a gel directly in the cartilage defect.
- ChondroFiller liquid is simple to handle.
- ChondroFiller Liquid is supplied in an easy-to-use two-chamber syringe.
- The treatment method is cost-effective.
ChondroFiller® liquid is available in a range of volumes (1.0 ml, 1.5 ml and 2.3 ml) and is suitable for treatment of grade III or grade IV cartilage defects up to a size of 3 cm2.
Our claim: highest quality and safety for patients
ChondroFiller® liquid is used to fill and treat clearly localised cartilage damage in various joints, including the hip, shoulder, knee and ankle joints, as well as the metatarsophalangeal joint.
Since its market launch, ChondroFiller® liquid has been successfully implanted in more than 12,000 patients worldwide. The CE-certified medical device is the result of decades of research in collaboration with scientists and researchers from the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart.
ChondroFiller® liquid is implanted first as liquid, which then becomes a gel.
ChondroFiller® liquid is implanted either arthroscopically or minimally invasively, depending on the localisation and size of the defect.
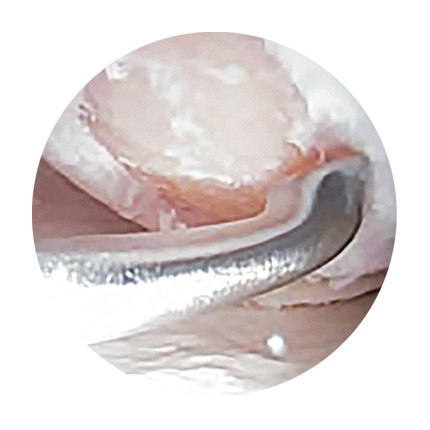
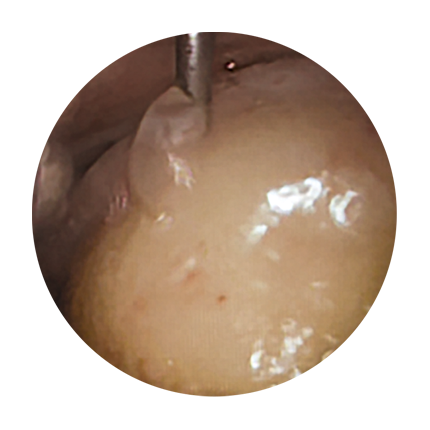
In the arthroscopy procedure, the position, degree and extent of the cartilage damage are determined. Here, any damaged cartilage tissue is removed at the same time. The defect zone is filled with the implant, which is initially liquid. It hardens after a few minutes after mixing of the components, resulting in complete coverage of the defect. See also Figures 1 to 4.
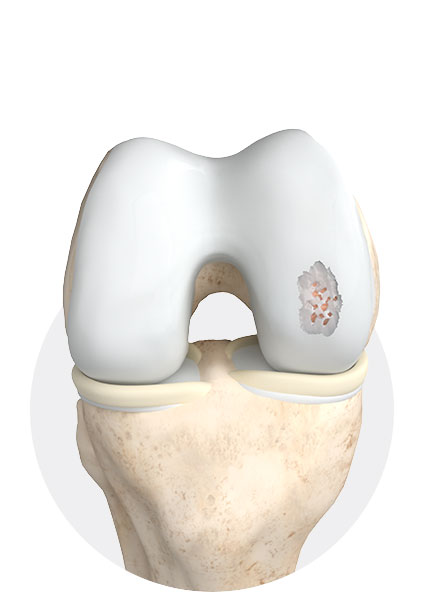
1
Cartilage damage grade 3-4
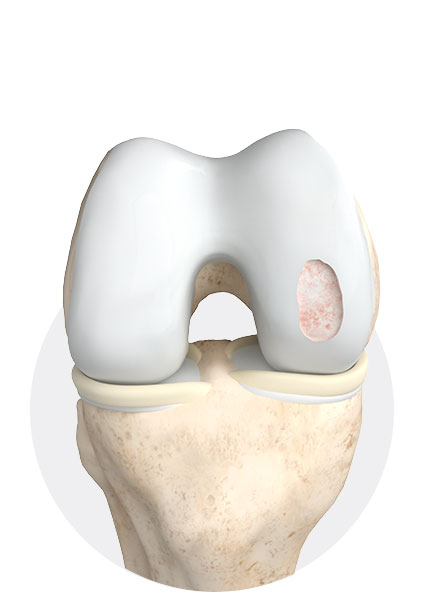
2
Cleaning of the defect
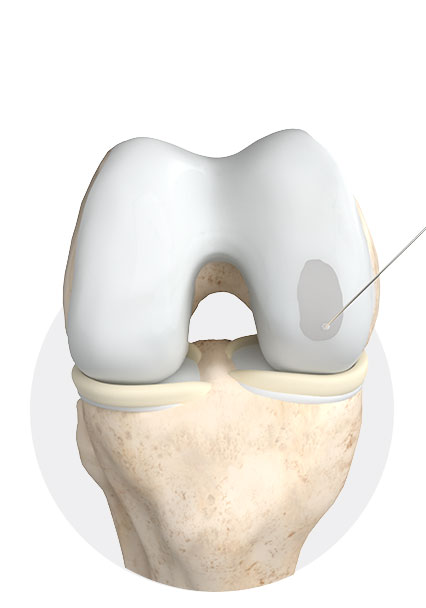
3
Filling of the defect with 3D matrix
4
Dimensionally stable regenerated material
Arthroscopy
ChondroFiller® liquid
MRT image of knee joint,
post-surgery
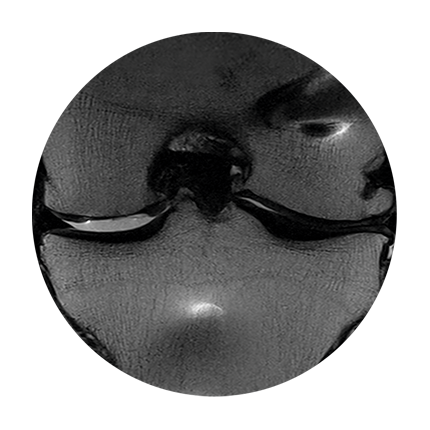
6 weeks post-surgery
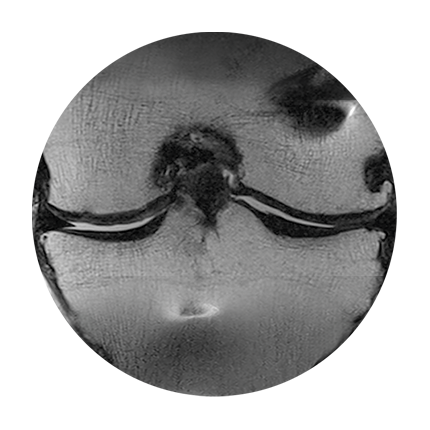
6 weeks post-surgery
